Background:
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only potentially curative treatment for patients with refractory/relapsed (R/R) acute myeloid leukemia (AML). However, the long-term survival results are suboptimal. Optimization of the conditioning regimen is needed to eradicate leukemia blasts and reduce early relapse. In this study, we made refinements in the double alkylators based conditioning regimen, namely MCBC (the combination of melphalan, cladribine, busulfan, and cyclophosphamide). We aim to investigate the efficacy and safety of MCBC conditioning regimen for allo-HSCT in R/R AML patients.
Methods: This prospective multi-center clinical trial was conducted in 13 tertiary hospitals in China, and enrolled R/R AML patients who underwent allo-HSCT from July 2020 to October 2022 (ChiCTR Registration ID: ChiCTR2000029936). The protocol was approved by institutional review boards at participating centers. All patients enrolled received MCBC preconditioning regimen (MCBC group): melphalan 60mg/m2/d, day -9~-8, cladribine 5mg/m2/d, day -7~-5, busulfan 3.2mg/kg/d, day -5~-3, cyclophosphamide 30mg/kg/d, day -2~-1. Rabbit ATG was added in haploid-identical and unrelated-matched donor transplantation. FK506/CsA +short course MTX±MMF was mostly used to prevent graft-versus-host disease (GVHD). We also retrospectively collected data from R/R AML patients who proceeded to allo-HSCT using the classical Bu/Cy2 based conditioning regimen, including Bu/Cy/Flu/Ara-C, Bu/Cy/Flu/IDA, et al, between Nov 2017 to Feb 2022 as history control (Bu/Cy2 group). Probabilities of OS, RFS were calculated by Kaplan-Meier method. A landmark survival analysis was performed.
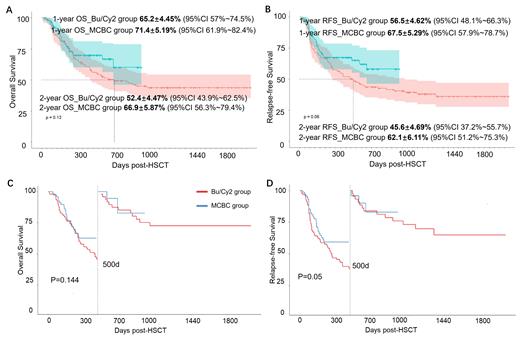
Results and Discussion: This multicenter MCBC trial prospectively enrolled 89 R/R AML patients, with median age 36 (ranged 11-59 years). Only 39.33% (35/89) patients achieved hematology remission at HSCT. Haplo-identical-donor HSCT (HID-HSCT) was the predominance HSCT type (n=62, 69.67%). Mucositis was the main reported regimen-related toxicity, mostly were mild and well tolerated. No graft failure was documented. All patients achieved hematology complete remission (CR) (100%) and 85.39% achieved MRD clearance on reconstitution day, indicating profound anti-leukemia capacity of MCBC regimen. The incidence of II to IV acute GVHD was 50.56%, with severe aGVHD (III to IV) only 8.99%. In comparison to the history control group, MCBC and Bu/Cy2 group were similar for age, gender, disease status, HSCT type. Notably, the incidence rate of mucositis (65.17% vs 63.48%, p=0.43) and diarrhea (87.64% vs 82.6%, p=0.57) were comparable in both groups. The median follow-up post HSCT was 431.29 days (95%CI 437.34~570.65) in MCBC group, and 1236 days (95%CI 1169.32~1302.68) in Bu/Cy2 group, respectively. The estimated one-year overall survival (OS), relapse-free survival (RFS) in MCBC and Bu/Cy2 group patients were 71.4±5.19% vs 65.2±4.45% (p=0.13), and 67.5±5.29% vs 56.2±4.62% (p=0.06, respectively. Notably, the survival advantages in MCBC group were expanded in the estimated two-year data. The estimated two-year overall survival (OS), relapse-free survival (RFS) in MCBC and Bu/Cy2 group patients were 66.9±5.87% vs 52.4±4.47%, and 62.1±6.11% vs 45.6±4.69%. We furtherly performed landmark survival analysis, and the result demonstrated the relapse-free survival superiority in MCBC group within 500 days post-HSCT, compared to Bu/Cy2 group (p=0.05).
Conclusion: Our study confirms the excellent anti-leukemic capacity and good acceptable toxicity of MCBC conditioning regimen in R/R AML.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal